RESULTS FROM THE PHASE 1
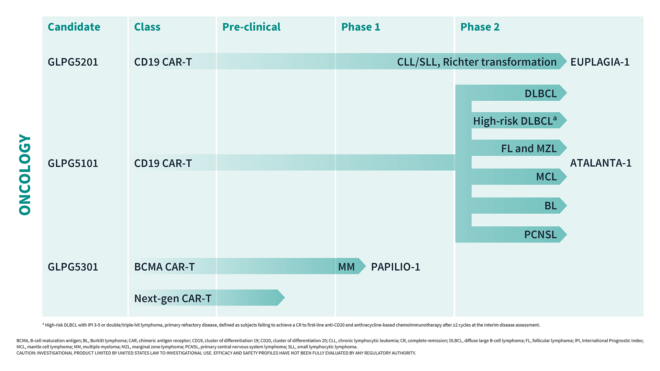
ATALANTA-1 TRIAL
SEVEN-DAY VEIN-TO-VEIN POINT-OF-CARE–MANUFACTURED CD19 CAR T-CELL THERAPY (GLPG5101) IN RELAPSED/REFRACTORY NON-HODGKIN LYMPHOMA (NHL)
Oral Presentation
Wednesday, 17 April, 12:57 – 13:06
Hall 2
Marie José Kersten1, Kirsten Saevels2, Sophie Servais3, Yves Beguin3, Joost S. P. Vermaat4, Eva Santermans5, Stavros Milatos6, Maike Spoon7, Marte C. Liefaard7, Claire Vennin7, Margot J. Pont7, Anna D.D. van Muyden7, Maria T. Kuipers1, Sébastien Anguille2
1University of Amsterdam, Amsterdam, Netherlands, 2Antwerp University Hospital, Antwerp, Belgium, 3Centre Hospitalier Universitaire de Liège, Liège, Belgium, 4Leiden University Medical Center, Leiden, Netherlands, 5Galapagos NV, Mechelen, Belgium, 6Galapagos GmbH, Basel, Switzerland, 7Galapagos BV, Oegstgeest, Netherlands