Galapagos | Booth F03
EHA Congress 2024
We are united around our mission to redefine the future of cancer treatment. Our goal is to help people facing cancer live longer, better lives. Our hope is fueled by the dream of a future free from cancer.
Pioneering for patients
Who we are
We are scientists, entrepreneurs, and pioneers united around a single purpose: to transform patient outcomes through life-changing science and innovation for more years of life and quality of life for people around the world. We focus on the key therapeutic areas of immunology and oncology, where we have developed deep scientific expertise in multiple drug modalities, including small molecules and cell therapies.
Redefining the Future of Cancer Treatment
Cancer is a complex disease with no one-size-fits-all solution. At Galapagos, our dedication lies in taking a holistic approach to tackle cancer. We’re at the forefront of developing innovative tactics that target cancer from multiple angles.
Our approach involves a diverse set of tools and strategies, including small molecules, antibody-based biological therapies, novel T-cell therapies, ingenious manufacturing technologies, and various other pioneering approaches to address the complexity of cancer.
Our focus in oncology
Delivering more years of life and improving the quality of life

Non-Hodgkin lymphoma (NHL)

Chronic lymphocytic leukemia (CLL) and Richter transformation (RT)
Multiple myeloma (MM)
Our innovative approach to
T-cell manufacturing near the patient
Galapagos’ decentralized, innovative T-cell manufacturing platform consists of:
- an end-to-end xCellit® workflow management and monitoring software system,
- a decentralized, functionally closed, automated manufacturing platform for cell therapies (using Lonza’s Cocoon®) and
- a proprietary quality control (QC) testing and release strategy.
The combination of these three core components offers the potential for the administration of a fresh product with a vein-to-vein time of 7 days (i.e. the time between T-cell collection and T-cell infusion), and greater physicians’ oversight throughout the process.
The Cocoon® Platform is a registered trademark of Lonza Group AG
Novel manufacturing technology
We are committed to decentralized manufacturing of
personalized cell therapies near patients
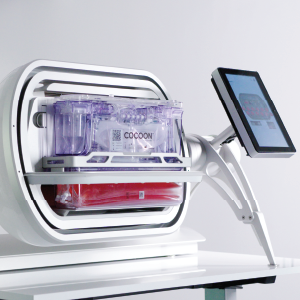
Novel decentralized
manufacturing model
Galapagos’ decentralized T-cell manufacturing platform, consists of an xCellit® workflow management and monitoring software, a manufacturing platform for cell therapies (using Lonza’s Cocoon®) and a proprietary quality control (QC) testing and release strategy
Greater physicians’
oversight
Enables clinicians to administer fresh, fit T-cells without complex logistics or cryopreservation and provides greater oversight for physicians during the process

Faster solutions
for patients
Ability to reduce the average vein-to-vein time (i.e. time between T-cell collection and T-cell infusion) from months to days
Our scientific presence at EHA 2024
New preliminary Phase 1/2 data for ATALANTA-1 in R/R NHL
SEVEN-DAY VEIN-TO-VEIN POINT-OF-CARE–MANUFACTURED CD19 CAR T CELLS (GLPG5101) IN RELAPSED/REFRACTORY NON-HODGKIN LYMPHOMA: RESULTS FROM THE PHASE 1/2 ATALANTA-1 TRIAL
Marie José Kersten
Author(s): Marie José Kersten, Kirsten Saevels, Sophie Servais, Evelyne Willems, Marte Liefaard, Stavros Milatos, Margot Pont, Claire Vennin, Eva Santermans, Anna van Muyden, Maria Kuipers, Sébastien Anguille, Joost Vermaat
(Abstract release date: 05/14/24) EHA Library. Kersten M. 06/13/2024; 422347; S243
Our clinical trials in Oncology
The ATALANTA-1 study is a phase 1/2 multicenter study evaluating the feasibility, safety, and efficacy of point-of-care manufactured GLPG5101 in subjects with relapsed/refractory B-cell non-Hodgkin lymphoma (rrNHL).
EudraCT 2021-003272-13
Key eligibility criteria
- rrNHL: DLBCL, MCL, FL, MZL, HR DLBCL, BL and PCNSL
- DLBCL, HR DLBCL, BL and PCNSL are allowed if primary refractory or relapse after 1 line of treatment
- FL, MZL and MCL are allowed after ≥ 2 lines of treatment
More information
The EUPLAGIA-1 study is a phase 1/2 study to evaluate the feasibility, safety, and efficacy of point-of-care manufactured GLPG5201 in subjects with relapsed/refractory chronic lymphocytic leukemia (rrCLL) or small lymphocytic lymphoma (rrSLL), including Richter transformation
EudraCT 2021-003815-25
Key eligibility criteria
- CD19+ rrCLL or rrSLL, Richter transformation included:
- CLL patients must have been exposed to at least 2 prior lines, including BTK inhibitors, BCL2 inhibitors, and/or PI3K inhibitors
- Richter patients who failed a BTK inhibitor are eligible regardless of number of prior therapy lines received
- No prior CD19-targeted therapies
More information
The PAPILIO-1 study is a phase 1/2 open-label, multicenter study evaluating the feasibility, safety, and efficacy of point-of-care manufactured GLPG5301 in subjects with relapsed/refractory multiple myeloma (rrMM)
EudraCT 2022-500782-27-00
Key eligibility criteria
- rrMM, plasma cell leukemia included, after at least 2 prior lines of therapy, including a proteasome inhibitor (PI), an immunomodulatory agent (IMiD), and anti-CD38 antibody treatment
- No prior BCMA-targeted therapies
More information
Our clinical pipeline in oncology
In our quest to advance cancer care, we focus on addressing the unique needs of patients with hematological malignancies.

Let’s connect!
Visit the Galapagos booth F03
Join our expanding
decentralized manufacturing network!
Given the initial results of our ongoing clinical trials, we are currently expanding our decentralized T-cell manufacturing network across the US and Europe.
Contact us for more information about the opportunities to join our ongoing and planned clinical studies.
GL-ONCO (CT)–202406-00003
Factsheet
Our commitment in oncology
Do you need everything in one place about our efforts in redefining the future of cancer treatments?